A 47-year-old male presents with chronic posterior knee pain that has progressively worsened over the past 18 months. The patient reports a dull, aching discomfort that intensifies with prolonged standing, stair climbing, and physical activity. He describes occasional episodes of sharp pain accompanied by a sensation of “catching” or “locking” in the knee joint. The patient notes morning stiffness lasting approximately 30 minutes and intermittent swelling around the posterior aspect of the knee. His medical history includes recreational basketball playing in his youth and a remote history of a knee sprain approximately 20 years ago. Conservative management with over-the-counter anti-inflammatory medications has provided minimal relief. Physical examination reveals moderate effusion, reduced range of motion, and crepitus with knee flexion and extension.
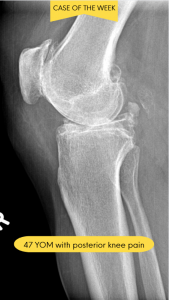

Based on advanced imaging studies and clinical correlation, the patient was diagnosed with osteochondromatosis secondary to degenerative joint disease (DJD). The radiology report revealed multiple intra-articular loose bodies within the posterior compartment of the knee joint, consistent with osteochondral fragmentation. Radiographic findings demonstrated moderate joint space narrowing, marginal osteophyte formation, and subchondral sclerosis, all characteristic features of underlying degenerative arthropathy. The presence of numerous calcified or ossified loose bodies confirmed the diagnosis of secondary osteochondromatosis resulting from progressive joint degeneration.
For practitioners seeking expertise in complex musculoskeletal imaging, consultation with specialists who hold credentials such as DACBR (Diplomate of the American Chiropractic Board of Radiology) can provide invaluable diagnostic clarity. These board-certified radiologists possess advanced training in interpreting subtle imaging findings that may be overlooked in standard evaluations.
Osteochondromatosis, also referred to as synovial osteochondromatosis when originating from synovial tissue, represents a condition characterized by the presence of multiple loose bodies within a joint space. These intra-articular fragments may be cartilaginous, calcified, or fully ossified, depending on the stage and chronicity of the condition.
The condition exists in two distinct forms:
Primary Osteochondromatosis (Synovial Chondromatosis): This rare form involves metaplastic transformation of the synovial membrane, leading to the formation of cartilaginous nodules within the joint lining. These nodules may detach and become free-floating loose bodies. Primary osteochondromatosis typically affects young to middle-aged adults and has a slight male predominance.
Secondary Osteochondromatosis: This more common form develops as a consequence of pre-existing joint pathology, most frequently degenerative joint disease, trauma, osteonecrosis, or osteochondritis dissecans. In secondary osteochondromatosis, cartilage fragments and bony debris break away from damaged articular surfaces or osteophytes, creating multiple loose bodies within the joint space.
Feature | Primary Osteochondromatosis | Secondary Osteochondromatosis |
Etiology | Synovial metaplasia (unknown cause) | Pre-existing joint disease (DJD, trauma, OCD) |
Age of Onset | 20-40 years | 40+ years (typically older) |
Gender | Slight male predominance | Equal distribution or slight male predominance |
Synovial Changes | Proliferative, cartilage-forming | Reactive inflammation |
Loose Body Characteristics | Uniform size, similar appearance | Variable sizes, irregular shapes |
Radiographic Pattern | Multiple rounded, similar densities | Mixed appearance with degenerative changes |
Associated Findings | Usually no pre-existing arthritis | Joint space narrowing, osteophytes, sclerosis |
Treatment Approach | Loose body removal + synovectomy | Loose body removal (synovectomy not required) |
Recurrence Risk | 5-15% (if synovectomy incomplete) | Low (unless DJD progresses) |
Malignant Potential | Rare (less than 5% to chondrosarcoma) | Essentially none |
The knee joint is the most commonly affected site, accounting for approximately 60 to 70% of cases, followed by the hip, elbow, and shoulder. The presence of loose bodies can perpetuate a cycle of mechanical irritation, synovial inflammation, and progressive joint damage if left untreated.
When complex cases arise, obtaining a second opinion from specialized diagnostic imaging consultants can significantly impact treatment planning and patient outcomes. Modern healthcare increasingly recognizes the value of subspecialty expertise in musculoskeletal radiology for challenging diagnostic scenarios.
Understanding osteochondromatosis requires familiarity with normal joint architecture. Synovial joints consist of several key components:
Joint Component | Function | Relevance to Osteochondromatosis |
Articular Cartilage | Smooth, low-friction surface for joint movement | Source of loose bodies when damaged |
Synovial Membrane | Produces synovial fluid for lubrication and nutrition | Site of metaplasia in primary form; becomes inflamed in response to loose bodies |
Joint Capsule | Fibrous tissue surrounding the joint space | Contains loose bodies within joint |
Synovial Fluid | Reduces friction and nourishes cartilage | Provides nutrition for loose body growth |
Subchondral Bone | Bone layer beneath articular cartilage | Contributes bony fragments in secondary form |
In Primary Osteochondromatosis:
The pathogenesis involves synovial metaplasia, where normal synovial cells undergo abnormal differentiation into cartilage-producing cells. This process, triggered by unknown factors possibly involving genetic mutations or growth factor dysregulation, leads to the formation of cartilaginous nodules within the synovial membrane. These nodules receive nutrients from synovial fluid and may undergo endochondral ossification, transforming into bony loose bodies. As nodules increase in size, they may detach from the synovial surface, becoming intra-articular loose bodies that move freely within the joint space.
In Secondary Osteochondromatosis:
This form results from mechanical breakdown of joint structures. In degenerative joint disease, repetitive microtrauma and biomechanical stress cause progressive deterioration of articular cartilage. As cartilage fragments detach, they combine with bone debris from exposed subchondral surfaces and fractured osteophytes to create loose bodies. The synovial membrane responds with inflammation and increased fluid production, creating an environment where loose bodies can grow through synovial fluid nourishment. This perpetuates a vicious cycle: loose bodies cause additional synovial irritation, leading to more inflammation and further cartilage degradation.
Loose bodies typically migrate to areas of low pressure within the joint, often gravitating toward recesses and posterior compartments. In the knee, they frequently accumulate in the posterior capsule, suprapatellar pouch, or intercondylar notch. Their movement can cause sudden mechanical symptoms as they become transiently trapped between articular surfaces, explaining the characteristic “catching” or “locking” sensations patients experience.
The size and number of loose bodies vary considerably. They may range from tiny rice-grain-sized fragments to large masses several centimeters in diameter. A comprehensive radiology report will document the size, number, location, and degree of mineralization of these bodies, information crucial for treatment planning.
Osteochondromatosis presents with a variable clinical picture depending on the number, size, and location of loose bodies, as well as the severity of underlying joint pathology.
Symptom | Description | Clinical Significance |
Pain | Chronic, aching joint pain that worsens with activity and weight-bearing; deep and poorly localized | Reflects synovial inflammation and mechanical irritation |
Mechanical Symptoms | Intermittent catching, locking, or giving way of the joint | Hallmark feature; occurs when loose bodies wedge between articular surfaces |
Swelling and Effusion | Visible joint swelling and palpable fluid accumulation | Results from chronic synovial irritation and increased fluid production |
Reduced Range of Motion | Gradually diminishing joint mobility | Due to progressive degeneration and mechanical blockage |
Crepitus | Grinding, clicking, or popping sensations with movement | Reflects both degenerative changes and intra-articular debris |
Stiffness | Morning stiffness or stiffness after inactivity (30 minutes to several hours) | Common in inflammatory and degenerative joint conditions |
Clinicians may observe visible joint swelling, palpable effusion (positive ballottement test in the knee), tenderness along joint lines, restricted range of motion, crepitus during passive motion, and occasional palpable loose bodies in superficial locations. In the knee, McMurray’s test or Apley’s compression test may elicit pain. Muscle atrophy of surrounding musculature may develop due to chronic pain and disuse.
While clinical presentation raises suspicion, definitive diagnosis requires imaging confirmation. Radiographs reveal radio-opaque loose bodies with varying degrees of mineralization, along with degenerative changes such as joint space narrowing, osteophyte formation, and subchondral sclerosis. Advanced imaging with MRI or CT provides superior visualization of cartilaginous loose bodies not yet calcified, assessment of articular cartilage integrity, and evaluation of synovial proliferation.
For practitioners managing complex musculoskeletal cases, partnering with Diagnostic Imaging Consultants who specialize in chiropractic radiology ensures comprehensive evaluation and accurate interpretation of subtle imaging findings that may influence clinical decision-making.
Healthcare providers in chiropractic and physical therapy settings frequently encounter patients with osteochondromatosis, making recognition and appropriate management essential for optimal patient outcomes.
Chiropractors and physical therapists serve as primary contact practitioners for many patients with musculoskeletal complaints. Recognizing the clinical patterns suggestive of osteochondromatosis (particularly mechanical symptoms, chronic effusion, and progressive functional decline) enables timely referral for appropriate imaging. When practitioners obtain DACBR consultation or request a second opinion on complex imaging findings, they demonstrate commitment to evidence-based practice and comprehensive patient care.
Intervention | Safety Level | Recommendations |
High-Velocity Manipulation | ⚠️ Caution/Contraindicated | Avoid or use extreme caution; risk of dislodging loose bodies and causing acute blockage |
Gentle Mobilization (Grades I-III) | ✓ Generally Safe | Recommended to maintain ROM without excessive joint stress |
Low-Impact Exercise | ✓ Recommended | Swimming, cycling, elliptical training minimize joint loading |
Strengthening Exercises | ✓ Recommended | Progressive resistance for periarticular muscles provides dynamic stability |
Range of Motion Exercises | ✓ Recommended | Gentle stretching within pain-free ranges prevents contractures |
Deep Tissue Work on Joint | ⚠️ Caution | Avoid aggressive techniques directly over affected joint |
Soft Tissue Techniques | ✓ Generally Safe | Myofascial release, trigger point therapy for secondary muscle tension |
Therapeutic Modalities | ✓ Safe | Ultrasound, electrical stimulation, ice/heat for symptomatic relief |
Key Exercise Principles:
Physical therapists play a crucial role in managing osteochondromatosis through carefully designed exercise programs. Progressive resistance training of periarticular muscles (quadriceps, hamstrings, hip abductors for knee involvement) provides dynamic joint stability and reduces abnormal mechanical stress. Balance and proprioceptive exercises enhance joint position sense and reduce injury risk.
Practitioners should educate patients about activity modification to minimize symptom exacerbation. This includes avoiding deep squatting, kneeling, high-impact activities, and movements that provoke catching or locking. Patients benefit from understanding their condition’s chronic nature and the importance of maintaining healthy body weight to reduce joint loading.
Chiropractic radiology specialists and physical therapists should maintain collaborative relationships with orthopedic surgeons for seamless referral when conservative management proves insufficient. Clear communication through comprehensive documentation, including detailed radiology reports and functional assessments, facilitates optimal interdisciplinary care.
Providers without advanced radiology training should not hesitate to request consultations with DACBR-certified specialists or other Diagnostic Imaging Consultants when imaging findings are complex or ambiguous. This collaborative approach ensures patients receive accurate diagnoses and appropriate treatment recommendations.
Management of osteochondromatosis requires individualized treatment planning based on symptom severity, functional impairment, number and size of loose bodies, and degree of underlying joint degeneration.
Indications: Mild to moderate symptoms, minimal functional limitation, small loose bodies, absence of mechanical locking, and patient preference for non-surgical approaches.
Aspect | Conservative Management | Surgical Management |
Best Candidates | Mild symptoms, small loose bodies, no locking | Severe symptoms, large/numerous loose bodies, mechanical locking |
Primary Goals | Symptom control, maintain function | Remove loose bodies, prevent further damage |
Pain Relief | Moderate (NSAIDs, injections, PT) | Significant and sustained |
Functional Improvement | Modest, maintenance focused | Substantial improvement in most cases |
Recovery Time | Immediate participation | 3 to 6 months to full activity |
Recurrence Prevention | Does not address underlying pathology | Removes source of mechanical symptoms |
Risks | Minimal (medication side effects) | Surgical risks, infection, stiffness |
Cost | Lower initial cost, ongoing expenses | Higher upfront cost, potentially lower long-term cost |
Success Rate | Variable, dependent on disease severity | 80 to 90% good to excellent outcomes |
Components of Conservative Management:
Pharmaceutical Management: Non-steroidal anti-inflammatory drugs (NSAIDs) reduce pain and inflammation. Intra-articular corticosteroid injections may provide temporary relief in cases with significant synovitis, though they do not address the loose bodies themselves.
Physical Therapy: As detailed previously, comprehensive rehabilitation programs focus on maintaining joint mobility, strengthening periarticular muscles, improving neuromuscular control, and modifying activities to reduce mechanical stress.
Assistive Devices: Bracing, taping, or orthotics may provide additional joint support and reduce aberrant movements that exacerbate symptoms.
Weight Management: For lower extremity joints, weight reduction significantly decreases joint loading and may slow degenerative progression.
Outcomes: Conservative management effectively controls symptoms in patients with mild osteochondromatosis but rarely addresses the underlying pathology. Patients require ongoing monitoring for symptom progression or development of mechanical complications.
Indications: Severe or progressive pain unresponsive to conservative care, recurrent mechanical locking or catching significantly impairing function, large or numerous loose bodies, evidence of progressive joint damage, and documented functional decline affecting quality of life or occupational activities.
Arthroscopic Loose Body Removal:
This minimally invasive approach represents the gold standard for treating symptomatic osteochondromatosis. Surgeons use small incisions and specialized instruments with camera guidance to visualize the joint interior and remove loose bodies. Advantages include reduced surgical trauma, faster recovery, lower infection risk, and ability to assess and address associated pathology such as meniscal tears or cartilage defects.
The procedure involves systematic exploration of all joint compartments and recesses to identify loose bodies, careful extraction to prevent fragmentation, synovectomy (removal of diseased synovial tissue) in primary osteochondromatosis, and treatment of associated lesions. Studies report high success rates with significant pain reduction and functional improvement in appropriately selected patients.
Open Arthrotomy:
For extensive disease with very large loose bodies, limited arthroscopic access, or concurrent procedures requiring open approaches, traditional open surgery may be necessary. While requiring longer recovery, open techniques provide complete visualization and access to all joint regions.
Synovectomy:
In primary osteochondromatosis, removing the diseased synovial membrane (synovectomy) is crucial for preventing recurrence, as the abnormal synovium continues producing cartilaginous nodules. This may be performed arthroscopically or through open technique depending on the extent of synovial involvement.
Definitive Joint Procedures:
In advanced cases with severe degenerative changes and irreversible joint damage, definitive procedures including total joint arthroplasty (replacement) or arthrodesis (fusion) may ultimately be necessary. These are typically reserved for end-stage disease when loose body removal alone cannot restore adequate function.
Following arthroscopic loose body removal, patients typically progress through structured rehabilitation phases:
Phase | Timeframe | Focus Areas | Key Activities |
Immediate Post-Op | 0 to 2 weeks | Reduce swelling, protect surgical sites | Gentle ROM, ice, compression, elevation |
Early Strengthening | 2 to 6 weeks | Progressive resistance, increase weight-bearing | Isometric exercises, partial weight-bearing activities |
Advanced Strengthening | 6 to 12 weeks | Sport-specific training, functional movements | Plyometrics, agility drills, return-to-sport preparation |
Return to Activity | 3 to 6 months | Unrestricted activity resumption | Full participation based on functional testing |
Physical therapists monitor for complications such as arthrofibrosis (excessive scar tissue formation), recurrent effusion, or inadequate strength recovery, adjusting rehabilitation protocols accordingly.
Prognosis depends significantly on the underlying cause and extent of joint damage. Secondary osteochondromatosis patients with mild to moderate underlying DJD who undergo loose body removal typically experience substantial symptom improvement, though the degenerative process continues. Primary osteochondromatosis has excellent outcomes following complete loose body removal and synovectomy, with recurrence rates of 5 to 15%. Long-term joint health requires ongoing attention to risk factor modification and maintenance of periarticular muscle strength.
Accurate diagnosis of osteochondromatosis requires differentiating it from other conditions presenting with similar clinical and radiographic features.
Condition | Key Clinical Features | Imaging Findings | Age Group | Distinguishing Factors |
Osteochondromatosis | Chronic pain, mechanical locking, effusion | Multiple rounded loose bodies, +/- DJD changes | 40+ (secondary), 20-40 (primary) | Multiple loose bodies, uniform or varied sizes |
Osteochondritis Dissecans | Focal pain, occasional locking | Single or few focal lesions, specific locations | Adolescents to young adults | Solitary lesion, characteristic locations |
DJD Without Loose Bodies | Chronic pain, stiffness | Joint space narrowing, osteophytes, sclerosis | 50+ | No loose bodies, less mechanical symptoms |
PVNS | Swelling, pain, limited ROM | Hemosiderin “blooming,” erosive changes | 20 to 40 | No calcified loose bodies, erosive bone changes |
CPPD (Pseudogout) | Acute inflammatory attacks | Linear/punctate cartilage calcification | 60+ | Chondrocalcinosis pattern, acute flares |
Meniscal Tear | Catching, locking, joint line pain | Meniscal signal changes on MRI | Any age, often athletic | Specific mechanism, no loose bodies |
Traumatic Fragments | Acute onset after trauma | Irregular, angular bone fragments | Any age | Recent trauma history, irregular fragments |
OCD involves focal separation of articular cartilage and underlying subchondral bone, typically affecting young, active individuals. It usually presents as a solitary lesion rather than multiple loose bodies. Imaging reveals a well-defined fragment within or separated from the articular surface, often in characteristic locations (lateral aspect of the medial femoral condyle in the knee). Unlike osteochondromatosis, OCD typically lacks diffuse degenerative changes unless longstanding and untreated.
Primary synovial chondromatosis can occasionally undergo malignant transformation to synovial chondrosarcoma, though this is rare (less than 5% of cases). Concerning features suggesting malignant transformation include rapid symptom progression, soft tissue mass extending beyond joint capsule, irregular mineralization patterns, bone invasion on imaging, and recurrence after appropriate surgical treatment. Any suspicious findings warrant prompt orthopedic oncology consultation and consideration of biopsy. A detailed radiology report from experienced subspecialists helps identify these concerning features.
Not all DJD cases develop osteochondromatosis. Patients with isolated degenerative changes without loose bodies typically lack the characteristic mechanical catching and locking symptoms. Imaging confirms degenerative features but absence of intra-articular loose bodies. These patients may benefit from conservative management without surgical intervention.
PVNS, a proliferative synovial disorder causing joint swelling and pain, can mimic osteochondromatosis clinically. However, PVNS typically demonstrates hemosiderin deposition creating characteristic “blooming artifact” on MRI gradient-echo sequences and erosive bone changes not seen in typical osteochondromatosis. PVNS rarely produces calcified loose bodies.
CPPD, commonly called pseudogout, causes acute inflammatory arthritis with calcification of cartilage (chondrocalcinosis). While both conditions show calcific densities on radiographs, CPPD typically demonstrates linear or punctate calcification within hyaline cartilage and fibrocartilage rather than discrete intra-articular loose bodies. Clinical presentation differs with acute inflammatory flares in CPPD versus chronic mechanical symptoms in osteochondromatosis.
Patients with meniscal tears or ligament injuries report mechanical symptoms similar to osteochondromatosis, including catching, locking, and giving way. However, these soft tissue injuries typically have specific traumatic mechanisms and demonstrate characteristic findings on MRI without the presence of loose bodies. McMurray’s test and Lachman’s test help differentiate meniscal and ligament pathology respectively.
Acute trauma can create intra-articular bone fragments mimicking osteochondromatosis. Clinical history of significant trauma, acute symptom onset, and imaging showing irregular, angular fracture fragments rather than smooth, rounded loose bodies help distinguish traumatic fragments from osteochondromatosis.
Every day, chiropractors face the same frustration: imaging reports that miss what matters. General radiologists weren’t trained in your world; they don’t understand subluxations, joint dysfunction, or the biomechanical findings that drive your treatment decisions.
The result? Delayed care. Uncertain patients. Cases that stall when they should be progressing.
The Kinetic Radiology Difference: Chiropractors Reading for Chiropractors
Our board-certified DACBRs aren’t just radiologists. We’re chiropractors who chose to specialize in musculoskeletal imaging. We speak your language because we’ve stood where you stand.
Reports You Can Act On Immediately – No vague findings. No irrelevant details. Just the specific insights that guide your next adjustment, your treatment plan, and your patient conversations.
Same-Day Turnaround – Your patients don’t want to wait days wondering what’s wrong. Neither should you. Get clarity fast so care never stalls.
Documentation That Protects Your Practice – Whether it’s insurance requirements, legal protection, or patient records, our reports give you the clinical backing you need.
Confidence That Builds Your Reputation – When patients see you consulting with specialized radiologists, they recognize your commitment to excellence. That trust turns into loyalty, referrals, and five-star reviews.
Think about the last complex case you handled. Did the radiology report actually help you—or did you have to fill in the gaps yourself?
Now imagine having a DACBR partner who catches the subtle findings, flags the red flags, and gives you confidence in every diagnosis.
No commitment. No risk. Just submit your next challenging case and experience what specialized chiropractic radiology can do for your clinical confidence and patient outcomes.
Questions? Call us at 321 325 0096 or email at support@kineticradiology.com
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.
A bone infarction is localized death of bone marrow due to loss of blood supply, commonly seen in patients with sickle cell disease.
A bone infarction occurs when the blood supply to bone marrow is interrupted, leading to ischemic necrosis. In sickle cell disease, this results from sickled red blood cells obstructing small intramedullary vessels. These blockages cause repeated episodes of ischemia and reperfusion injury, which destroy marrow architecture and lead to chronic bone pain.
DACBRs and diagnostic imaging consultants often identify bone infarctions incidentally when interpreting MRIs or X-rays ordered for pain evaluation. Radiographically, chronic infarctions may appear as serpiginous, sclerotic lesions with central lucency. MRI is the modality of choice—it can detect early marrow changes before X-ray findings emerge. The “double line sign” on T2-weighted images is characteristic.
For chiropractors and PTs, understanding this process is key. Bone infarctions are not mechanical injuries, so aggressive manual therapy may aggravate symptoms. Instead, clinicians should integrate radiology report findings into treatment planning, ensuring interventions respect the bone’s healing phase. Collaboration with a DACBR (Doctor of Chiropractic, Board Certified Radiologist) helps confirm diagnosis and prevent mismanagement.
AVN affects subchondral bone near joint surfaces, while bone infarction involves the medullary cavity of long bones.
Though both involve ischemic bone death, avascular necrosis (AVN) and bone infarction differ by anatomic location and clinical impact. AVN affects subchondral bone adjacent to a joint, commonly in the femoral head, and can lead to joint collapse. Bone infarctions, by contrast, affect the marrow cavity and generally spare the articular surface.
From a diagnostic imaging perspective, this distinction is crucial. MRI interpreted by a DACBR can identify subchondral collapse or the crescent sign typical of AVN, versus serpiginous medullary signal patterns in infarction. Radiology reports often use descriptors such as “medullary infarction” or “osteonecrosis” to clarify this difference.
Clinically, chiropractors and PTs will note that AVN leads to stiffness, restricted range of motion, and mechanical pain—while infarction produces deeper, diffuse, non-mechanical pain. Understanding this difference guides care: joint-preserving rehab strategies for AVN versus supportive, non-load interventions for infarction.
Collaboration with diagnostic imaging consultants ensures accurate identification and safe progression of rehabilitation plans. Reviewing radiology reports with a DACBR’s insight can help clinicians identify subtle differences and avoid inappropriate care in ischemic bone.
Sickle-shaped red blood cells block bone microcirculation, leading to repeated ischemic injury and marrow necrosis.
In sickle cell disease, hemoglobin S distorts red blood cells into rigid, crescent shapes that can’t pass smoothly through capillaries. Within bone marrow’s sluggish microcirculation, these cells cluster and cause microvascular occlusion, depriving tissues of oxygen. Over time, this leads to bone infarctions, especially in weight-bearing long bones.
These recurrent ischemic episodes are a hallmark of sickle cell crises—periods of acute pain due to widespread microinfarction. As infarcted areas heal, they form dense sclerosis and irregular bone patterns visible on X-rays and MRI. Radiologists (and DACBRs) recognize these chronic findings as serpiginous medullary sclerosis.
For chiropractors and PTs, this mechanism explains why SCD-related pain may mimic musculoskeletal disorders but doesn’t respond predictably to joint manipulation or soft tissue work. Understanding radiology report terminology—like “medullary infarction” or “marrow signal alteration”—helps clinicians interpret these cases accurately and refer appropriately.
A multidisciplinary approach involving diagnostic imaging consultants, hematologists, and rehabilitation specialists provides the safest path forward for long-term mobility and pain control.
The femur, humerus, and tibia are most frequently affected by bone infarctions in sickle cell disease.
Bone infarctions in sickle cell disease most commonly occur in long tubular bones such as the femur, humerus, and tibia due to their high marrow volume and relatively poor collateral circulation. Flat bones like the pelvis or ribs may also be involved but less frequently.
Radiology reports often describe infarcts in the diaphyseal or metaphyseal regions, sparing the subchondral surfaces. On MRI, diagnostic imaging consultants may note serpiginous T1 and T2 signal changes in the marrow cavity—often bilateral and symmetric. Chronic lesions can calcify or cause cortical thinning, predisposing to fractures.
For chiropractors and physical therapists, recognizing these patterns is essential when treating lower extremity or shoulder pain. Pain localized to these bones may indicate ischemic pathology rather than joint dysfunction. Coordination with a DACBR ensures accurate correlation between imaging findings and clinical presentation, reducing the risk of inappropriate loading or manipulation on structurally compromised bone.
In weight-bearing regions like the femur, even minor stress can precipitate pain crises, so gradual weight-bearing progression is critical during rehabilitation.
They present with deep, throbbing bone pain, swelling, and limited motion, often unrelated to injury or activity.
Clinically, bone infarctions in sickle cell patients manifest as deep, dull, or throbbing pain, often localized to long bones or joints but without a clear history of trauma. Pain tends to worsen during sickle crises, when deoxygenation increases vascular blockage. Swelling and warmth may occur, mimicking infection or inflammation.
A key differentiator for chiropractors and PTs is that this pain is not mechanical—it doesn’t fluctuate with posture or activity. Motion testing may reveal pain but not necessarily restriction. During acute episodes, the bone is vulnerable to microfracture, so heavy manual therapy or high-force mobilization should be avoided.
Radiologically, DACBRs identify these lesions through MRI findings showing medullary edema or chronic serpiginous borders. Reviewing the radiology report for these indicators allows clinicians to tailor conservative care appropriately.
For physical therapists, gentle range of motion, hydrotherapy, and pain modulation (e.g., TENS, heat) are recommended once acute pain subsides. Chiropractors should focus on supportive care and interprofessional communication with hematology or radiology specialists for safe co-management.
Partnering with a DACBR teleradiology service provides more than just a second opinion; it offers a significant return on investment:
Speed: Get expert reports in hours, not days.
Expertise: Access board-certified specialists without having to hire them.
Convenience: The entire process is handled online from your office.
Clarity: Receive clear, concise reports that are clinically relevant to chiropractic care, not generic medical reports.
Posted onTrustindex verifies that the original source of the review is Google. Kinetic radiology has been an absolute game changer in speed of reports and detailed reports. Any other doctors I send my reports to are amazed at the detail and the pathology that gets picked up. This is my one and only radiologist group, im thrilled.Posted onTrustindex verifies that the original source of the review is Google. Rishi provides an outstanding service—fast, reliable, and incredibly reassuring. He’s quick to respond, efficient in his work, and always takes the time to address any concerns with clarity and professionalism. I highly recommend his services to anyone looking for a dependable DACBR.Posted onTrustindex verifies that the original source of the review is Google. Prompt efficient service that is thorough and clear. Spinal information is top notch and I've had patients discover kidney stones and possible issues with a hip joint replacement loosening as incidental findings that supported both me and the patient above expectations.Posted onTrustindex verifies that the original source of the review is Google. Quick, accurate, and easy to work with. My new radiology team!Posted onTrustindex verifies that the original source of the review is Google. Excellent, timely reads. Invaluable for CBCTPosted onTrustindex verifies that the original source of the review is Google. Best turnaround time and thorough reports out of any radiologist I’ve seen or worked with!Posted onTrustindex verifies that the original source of the review is Google. Very detailed reports and quick service. Highly recommendedPosted onTrustindex verifies that the original source of the review is Google. Fast turn around time for the radiology reports! Thank you for making this process as seamless as possible!Posted onTrustindex verifies that the original source of the review is Google. I am a NUCCA chiropractor located in Wauankee Wisconsin and I can tell you Dr. Rishi is the only radiologist I’d work with. Sure there are many others in my area but when you want the best you go to the best. He is very easy to work with and always fast to respond and report. 100% recommend.Posted onTrustindex verifies that the original source of the review is Google. Kinetic Radiology is great! They were able to read and get a report written immediately. They are my go to company for any and all images that I need read!Load more
We service all 50 U.S. States, including the following States and Cities listed below.
Copyright 2024 Kinetic Radiology All Rights Reserved
Website Privacy | Terms of UseReceive timely resources to keep you and your practice on the cutting edge of Chiropractic Radiology.
Copyright 2024 Kinetic Radiology
All Rights Reserved
Receive timely resources to keep you and your practice on the cutting edge of Chiropractic Radiology.