Calcium pyrophosphate deposition disease (CPPD) is a common crystal arthropathy caused by deposition of calcium pyrophosphate dihydrate crystals in hyaline cartilage, fibrocartilage, synovium, and periarticular soft tissues. Chondrocalcinosis is the radiographic term describing visible calcification within cartilage and is the most recognizable imaging manifestation of CPPD, though not all patients with CPPD have radiographic chondrocalcinosis and not all chondrocalcinosis is symptomatic CPPD arthritis. CPPD can present as asymptomatic chondrocalcinosis, acute “pseudogout” attacks, chronic inflammatory arthritis mimicking osteoarthritis or rheumatoid arthritis, or atypical presentations such as spinal or tendon involvement.
For chiropractors, primary care providers, and urgent care clinicians, CPPD is highly relevant because it frequently presents as acute or chronic joint pain in older adults and is often first identified on plain radiographs ordered for musculoskeletal complaints. Diagnostic imaging consultants, especially a board‑certified DACBR, can help distinguish CPPD‑related chondrocalcinosis from other causes of intra‑articular calcification and advise on the need for further imaging, synovial fluid analysis, and medical referral. When imaging findings are subtle, atypical, or clinically discordant, a radiology second opinion can be critical for accurate diagnosis and appropriate management.
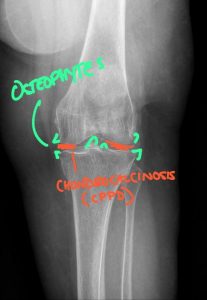
Calcium pyrophosphate deposition disease (CPPD) involving the knee, characterized by chondrocalcinosis within the menisci and articular cartilage. CPPD results in calcium pyrophosphate crystal deposition that promotes cartilage degeneration and secondary osteoarthritic changes, including marginal osteophyte formation and joint space degeneration.
CPPD is a crystal deposition arthropathy in which calcium pyrophosphate crystals form and accumulate in articular and periarticular tissues, triggering local inflammation and joint damage. The reference standard for diagnosis is identification of positively birefringent, rhomboid‑shaped calcium pyrophosphate crystals in synovial fluid under polarized light microscopy. However, in everyday practice, diagnosis is often presumed when patients with compatible symptoms show characteristic radiological findings of chondrocalcinosis.
Important terminology distinctions:
Chondrocalcinosis: Radiographic description of calcification in articular cartilage or fibrocartilage; not synonymous with clinical CPPD disease.
CPPD disease: Clinical spectrum of manifestations caused by CPP crystal deposition, including asymptomatic chondrocalcinosis, acute CPP crystal arthritis (“pseudogout”), chronic CPP crystal inflammatory arthritis, and OA‑like CPPD.
CPP crystal deposition is linked to imbalances in extracellular inorganic pyrophosphate metabolism and often associated with aging, joint degeneration, and metabolic conditions such as hyperparathyroidism, hemochromatosis, hypomagnesemia, and hypophosphatasia. In some patients, metabolic workup may reveal a correctable underlying disorder.
CPPD is the third most common inflammatory arthritis after gout and rheumatoid arthritis, with prevalence increasing significantly with age. Radiographic chondrocalcinosis is uncommon before age 50 but may be found in up to 15–20% of individuals over 80, especially in weight‑bearing joints such as the knees. CPPD affects men and women roughly equally, though specific patterns may vary by joint and phenotype.
Established risk factors include:
Advanced age
Osteoarthritis and prior joint trauma or surgery (e.g., meniscectomy)
Metabolic/endocrine disorders: hyperparathyroidism, hemochromatosis, hypomagnesemia, hypophosphatasia, chronic kidney disease
Genetic predisposition in some families, particularly with early‑onset or polyarticular disease
For clinicians, identification of CPPD—especially in younger patients (<60) or those with widespread chondrocalcinosis—should prompt consideration of metabolic and genetic evaluation. Diagnostic imaging consultants can flag imaging patterns that suggest secondary causes, supporting targeted laboratory work‑ups.
CPPD has a broad clinical spectrum, which can make it a diagnostic challenge.
Asymptomatic Chondrocalcinosis
Many patients with radiographic chondrocalcinosis are entirely asymptomatic, with calcifications discovered incidentally during imaging for unrelated complaints. These cases do not require specific treatment but may warrant documentation and, in some settings, metabolic screening.
Acute CPP Crystal Arthritis (“Pseudogout”)
The classic symptomatic presentation is acute CPP crystal arthritis, historically termed “pseudogout,” because it resembles gout clinically but involves CPP crystals rather than monosodium urate. Patients present with:
Sudden onset of severe joint pain, swelling, warmth, and erythema
Often monoarticular or oligoarticular, most commonly involving the knee, wrist, ankle, or shoulder
Attacks can be triggered by illness, surgery, trauma, or metabolic shifts
Acute CPP crystal arthritis is self‑limited, typically resolving over days to weeks with appropriate anti‑inflammatory treatment.
Chronic CPPD Arthritis
Chronic CPP crystal inflammatory arthritis may mimic rheumatoid arthritis or osteoarthritis. Features include:
Persistent or recurrent joint pain and stiffness
Swelling, reduced range of motion, and sometimes inflammatory signs
Involvement of knees, wrists, MCP joints, hips, shoulders, and spine
Radiographs may show joint space narrowing, osteophytes, subchondral cysts, and chondrocalcinosis—sometimes with more severe degeneration than expected for age
Atypical and Spinal Manifestations
CPPD can also present as:
CPPD‑related OA: Osteoarthritis pattern in atypical sites (e.g., radiocarpal or MCP joints) with chondrocalcinosis.
Crowned dens syndrome: CPP crystal deposition around the odontoid process causing acute neck pain, stiffness, fever, and elevated inflammatory markers; CT shows calcification around the dens.
Tendon and ligament calcification: Involving menisci, triangular fibrocartilage, Achilles tendon, or other periarticular structures.
Patients with neck pain, neurologic symptoms, or atypical calcifications on imaging may benefit from a radiology second opinion by a DACBR to differentiate CPPD from other causes such as DISH, OPLL, or degenerative changes.
Diagnostic imaging is central to recognizing chondrocalcinosis and assessing the extent of CPPD involvement.
Conventional Radiography
Conventional radiography remains the initial imaging test for suspected CPPD and is widely available in chiropractic, primary care, and urgent care settings. Classic radiographic features include:
Chondrocalcinosis: Linear or punctate calcifications within hyaline cartilage or fibrocartilage.
In hyaline cartilage (e.g., femoral condyles), CPPD appears as thin, linear, or band‑like densities parallel to the articular surface.
In fibrocartilage (e.g., menisci, triangular fibrocartilage of the wrist), it appears as irregular, punctate, or linear calcifications following the contour of the fibrocartilage.
Joint distribution: Knees, wrists, symphysis pubis, hips, elbows, shoulders, and spine are commonly affected.
Associated degenerative changes: Joint space narrowing, osteophytes, subchondral cysts, and sclerosis that may be more severe or in atypical locations compared to primary osteoarthritis.
Radiographs may underestimate CPPD burden—sensitivity for chondrocalcinosis detection in some studies was around 13% compared with 84% for ultrasound in the knee. For chiropractors and front‑line clinicians, identifying chondrocalcinosis on plain films should prompt consideration of CPPD and, when appropriate, consultation with diagnostic imaging consultants or a DACBR for confirmation and guidance on further evaluation.
Ultrasound
Musculoskeletal ultrasound has emerged as a highly sensitive tool for detecting CPPD deposits, particularly in the knees and wrists. Characteristic ultrasound findings include:
Thin hyperechoic bands parallel to the articular cartilage surface representing CPP deposits in hyaline cartilage.
Hyperechoic foci within fibrocartilage (e.g., menisci) and tendons, with or without posterior acoustic shadowing.
In one study, ultrasound detected chondrocalcinosis in 84.2% of CPPD cases compared with 13.2% for conventional radiography, with 100% specificity when synovial fluid crystal analysis was used as the reference standard. For clinicians working with diagnostic imaging consultants or DACBRs who also provide diagnostic ultrasound, this modality can substantially improve CPPD detection, especially in patients with normal or equivocal radiographs.
CT and Dual‑Energy CT
Conventional CT can depict cartilage and fibrocartilage calcifications with greater detail than radiography and is particularly useful in complex anatomical regions such as the spine and craniocervical junction. CT is the modality of choice for evaluating crowned dens syndrome, clearly demonstrating curvilinear calcifications around the odontoid process.
Dual‑energy CT (DECT), while more established in gout imaging, is being explored for differentiating CPP crystals from other types of calcifications, potentially improving specificity in crystal arthropathies. Currently, DECT remains more of a research and specialty tool than a first‑line test in most clinical settings.
MRI
MRI is less sensitive and specific for detecting CPPD compared to radiography, ultrasound, or CT, but it may reveal associated inflammatory changes and complications. MRI can show joint effusion, synovitis, bone marrow edema, and degenerative changes; CPP deposits may appear as low‑signal foci on all sequences but are not always conspicuous. MRI is useful when clinicians suspect CPPD discitis, spinal involvement, or when alternative diagnoses (e.g., infection, tumor, inflammatory arthropathy) are being considered. Interpretation of subtle CPPD manifestations on MRI is an area where a radiology second opinion from a DACBR or other musculoskeletal imaging specialist can be especially valuable.
Every day, chiropractors face the same frustration: imaging reports that miss what matters. General radiologists weren’t trained in your world; they don’t understand subluxations, joint dysfunction, or the biomechanical findings that drive your treatment decisions.
The result? Delayed care. Uncertain patients. Cases that stall when they should be progressing.
The Kinetic Radiology Difference: Chiropractors Reading for Chiropractors
Our board-certified DACBRs aren’t just radiologists. We’re chiropractors who chose to specialize in musculoskeletal imaging. We speak your language because we’ve stood where you stand.
Reports You Can Act On Immediately – No vague findings. No irrelevant details. Just the specific insights that guide your next adjustment, your treatment plan, and your patient conversations.
Same-Day Turnaround – Your patients don’t want to wait days wondering what’s wrong. Neither should you. Get clarity fast so care never stalls.
Documentation That Protects Your Practice – Whether it’s insurance requirements, legal protection, or patient records, our reports give you the clinical backing you need.
Confidence That Builds Your Reputation – When patients see you consulting with specialized radiologists, they recognize your commitment to excellence. That trust turns into loyalty, referrals, and five-star reviews.
Think about the last complex case you handled. Did the radiology report actually help you—or did you have to fill in the gaps yourself?
Now imagine having a DACBR partner who catches the subtle findings, flags the red flags, and gives you confidence in every diagnosis.
No commitment. No risk. Just submit your next challenging case and experience what specialized chiropractic radiology can do for your clinical confidence and patient outcomes.
Questions? Call us at 321 325 0096 or email at support@kineticradiology.com
Chondrocalcinosis is radiographic cartilage calcification often caused by calcium pyrophosphate deposition disease (CPPD), but not all chondrocalcinosis represents symptomatic CPPD.
Chondrocalcinosis is a descriptive radiology term that refers to visible calcification within hyaline cartilage or fibrocartilage on imaging, most often seen on plain radiographs or CT. The most common cause is calcium pyrophosphate deposition disease (CPPD), in which calcium pyrophosphate dihydrate crystals accumulate in articular and periarticular tissues and may trigger episodes of acute or chronic inflammatory arthritis. However, chondrocalcinosis can be asymptomatic, and some patients never develop clinical CPPD arthritis despite clear cartilage calcification. Conversely, CPPD can occasionally occur without obvious radiographic chondrocalcinosis, especially in early disease or in joints not well visualized on routine films. Diagnostic imaging consultants and DACBRs play a key role in recognizing chondrocalcinosis on X‑ray, differentiating it from other causes of intra‑articular calcification, and advising when clinical correlation or synovial fluid analysis is needed. When the relationship between imaging findings and symptoms is unclear, a radiology second opinion helps clarify whether chondrocalcinosis reflects active CPPD disease, incidental crystal deposition, or another process.
CPP crystals form due to disturbances in inorganic pyrophosphate metabolism, aging, joint degeneration, and associated metabolic or genetic conditions.
Calcium pyrophosphate crystals develop when there is excess inorganic pyrophosphate in the extracellular matrix of cartilage and synovial tissues, which then combines with calcium to form CPPD crystals. Aging and osteoarthritis are major contributors, as cartilage damage and altered chondrocyte function disrupt normal pyrophosphate regulation. Several systemic conditions—such as hyperparathyroidism, hemochromatosis, hypomagnesemia, hypophosphatasia, and chronic kidney disease—also promote CPP crystal formation by altering mineral metabolism or cartilage health. In some families, genetic variants affecting pyrophosphate transport or metabolism lead to early‑onset, polyarticular CPPD. These factors create an environment in which crystals deposit in cartilage, fibrocartilage, and soft tissues, eventually triggering inflammation in some individuals. Diagnostic imaging consultants may suggest metabolic screening when they see widespread or unusually severe chondrocalcinosis, and a DACBR providing a radiology second opinion can help correlate imaging patterns with potential underlying metabolic drivers.
CPPD most commonly affects the knees and wrists but can involve hips, shoulders, elbows, hands, spine, and other fibrocartilaginous structures.
The knees are the most frequently involved site for chondrocalcinosis, with CPP deposits often seen in menisci and hyaline cartilage on radiographs or ultrasound. Wrists are another classic location, where the triangular fibrocartilage complex and radiocarpal joint commonly show calcifications. Other typical sites include the symphysis pubis, hips, shoulders, elbows, ankles, and MCP joints, especially in chronic CPPD arthritis. CPPD can also affect the spine, particularly the cervical region, where calcification around the odontoid process (crowned dens syndrome) may cause acute neck pain and stiffness. Tendons and ligaments, such as the Achilles tendon or other periarticular structures, can demonstrate calcifications as well. Radiology reports from diagnostic imaging consultants and DACBRs frequently emphasize these characteristic sites and distributions, helping clinicians recognize CPPD patterns and differentiate them from degenerative or post‑traumatic calcifications. When unusual joints or atypical patterns are involved, a radiology second opinion is useful to confirm that CPPD is indeed the underlying cause.
CPPD is caused by calcium pyrophosphate crystals and often shows chondrocalcinosis on imaging, whereas gout is caused by monosodium urate crystals and has different crystal and imaging features.
Both CPPD and gout are crystal‑induced arthritides that cause acute inflammatory joint attacks, but they differ in crystal chemistry, risk factors, and imaging appearances. In gout, the culprit is monosodium urate crystals that form in the setting of hyperuricemia and deposit in synovial tissues and tophi; under polarized light microscopy, they appear needle‑shaped and strongly negatively birefringent. In CPPD, the crystals are calcium pyrophosphate dihydrate, rhomboid in shape, and weakly positively birefringent on polarized microscopy. Clinically, both can cause sudden painful joint swelling, but gout classically targets the first MTP joint, while CPPD more often affects knees, wrists, and larger joints. Imaging also differs: CPPD is strongly associated with chondrocalcinosis—linear or punctate cartilage calcification—while gout tends to show erosions with overhanging edges and tophaceous deposits without cartilage calcification. Dual‑energy CT can help distinguish urate from calcium‑containing crystals when needed. When the clinical picture is ambiguous, diagnostic imaging consultants and DACBRs can interpret subtle imaging differences and recommend synovial fluid analysis to definitively differentiate gout from CPPD, and a radiology second opinion can be especially helpful in complex or overlapping presentations.
Partnering with a DACBR teleradiology service provides more than just a second opinion; it offers a significant return on investment:
Speed: Get expert reports in hours, not days.
Expertise: Access board-certified specialists without having to hire them.
Convenience: The entire process is handled online from your office.
Clarity: Receive clear, concise reports that are clinically relevant to chiropractic care, not generic medical reports.
Posted onTrustindex verifies that the original source of the review is Google. Kinetic radiology has been an absolute game changer in speed of reports and detailed reports. Any other doctors I send my reports to are amazed at the detail and the pathology that gets picked up. This is my one and only radiologist group, im thrilled.Posted onTrustindex verifies that the original source of the review is Google. Rishi provides an outstanding service—fast, reliable, and incredibly reassuring. He’s quick to respond, efficient in his work, and always takes the time to address any concerns with clarity and professionalism. I highly recommend his services to anyone looking for a dependable DACBR.Posted onTrustindex verifies that the original source of the review is Google. Prompt efficient service that is thorough and clear. Spinal information is top notch and I've had patients discover kidney stones and possible issues with a hip joint replacement loosening as incidental findings that supported both me and the patient above expectations.Posted onTrustindex verifies that the original source of the review is Google. Quick, accurate, and easy to work with. My new radiology team!Posted onTrustindex verifies that the original source of the review is Google. Excellent, timely reads. Invaluable for CBCTPosted onTrustindex verifies that the original source of the review is Google. Best turnaround time and thorough reports out of any radiologist I’ve seen or worked with!Posted onTrustindex verifies that the original source of the review is Google. Very detailed reports and quick service. Highly recommendedPosted onTrustindex verifies that the original source of the review is Google. Fast turn around time for the radiology reports! Thank you for making this process as seamless as possible!Posted onTrustindex verifies that the original source of the review is Google. I am a NUCCA chiropractor located in Wauankee Wisconsin and I can tell you Dr. Rishi is the only radiologist I’d work with. Sure there are many others in my area but when you want the best you go to the best. He is very easy to work with and always fast to respond and report. 100% recommend.Posted onTrustindex verifies that the original source of the review is Google. Kinetic Radiology is great! They were able to read and get a report written immediately. They are my go to company for any and all images that I need read!Load more
We service all 50 U.S. States, including the following States and Cities listed below.
Copyright 2024 Kinetic Radiology All Rights Reserved
Website Privacy | Terms of UseReceive timely resources to keep you and your practice on the cutting edge of Chiropractic Radiology.
Copyright 2024 Kinetic Radiology
All Rights Reserved
Receive timely resources to keep you and your practice on the cutting edge of Chiropractic Radiology.